Frontiers |
您所在的位置:网站首页 › histogenous 翻译 › Frontiers |
Frontiers
 Xingbing Huang1 Xingbing Huang1 Qiu-Ling Lu1 Qiu-Ling Lu1 Xiu-Mei Zhu1 Xiu-Mei Zhu1 Yi-Bin Zeng1 Yi-Bin Zeng1 Yun Liu1 Yun Liu1 Hao-Ying Hu2*
1Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
2First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China Hao-Ying Hu2*
1Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
2First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Background: Oxidative stress may play an important role in the pathogenesis of schizophrenia (SCH), and there is considerable indirect evidence that hypoxia is closely related to SCH, but direct evidence of hypoxia in SCH has never been found. Methods:In this study, superoxide dismutase (SOD), venous blood gas, and Positive and Negative Syndrome Scale (PANSS) were examined in 40 SCH patients and compared with those of 40 healthy controls. The patients were treated with combination of atypical antipsychotics and Ditan Huayu Lishen decoction (a Chinese medicine decoction) and examined in the acute and stable period, respectively. Comparisons of indicators between two groups were performed using an independent-samples t-test, comparison of indicators between the acute and stable periods in the SCH group was performed using paired-samples t-test. Pearson correlation and multiple linear regression analyses were performed to investigate the relationships between the effect indicators. Results: Higher venous pH, PvO2, and fasting blood glucose levels and lower SOD, lactic acid, and PvCO2 levels were found in SCH patients compared with the control group; SOD was negatively correlated with the general psychopathology subscale score (PANSS-G), and PvO2 levels were closely related to venous pH in SCH and related to PvCO2 in the control group. It was also found that SOD activity showed no significant difference in acute and stable period, whereas PvO2 showed a downward trend, and venous pH was decreased significantly after treatment. Both the venous pH and PvO2 were higher in patients with SCH than that in healthy controls. Conclusion: It suggests that histogenous hypoxia and acid retention exist in relation to SCH, and there is an improvement of acid retention and a downward trend in histogenous hypoxia after combination treatment. Venous pH, PvO2, and PvCO2 are trait variables, but not state variables of SCH. The theory of histogenous hypoxia and acid retention can well explain the decrease in pH value and the increase in lactic acid in brain tissue of patients with SCH. Histogenous hypoxia and acid retention closely related to glucose metabolism. So they may play an important role in pathophysiology for SCH. IntroductionSchizophrenia (SCH) is a category of major mental health disorders of unknown etiology that encompasses significant abnormalities in cognitive, logical, emotional, behavioral, and other mental activities and leads to significant occupational and social function limitations (1). The lifetime prevalence of SCH was found to be 0.6% in a recent epidemiological survey of mental disorders in mainland China (2). Although genetic and environmental factors are thought to be involved in the pathogenesis of the disease, the cause of SCH remains unclear. There is increasing evidence, however, that oxidative stress may play an important role in the pathogenesis of SCH (3). People with SCH have been found to have impaired antioxidant defenses and experience increased oxidative damage (4, 5). Severe oxidative stress can lead to decreasing antioxidant content and an increase in the production of free radicals, leading to cell damage and even cell death (6). According to the meta-analysis results, the level of activity of erythrocyte superoxide dismutase (SOD) was reduced in acuter elapse of psychosis, drug-naive first-episode psychosis, stable medicated outpatients, and chronic inpatients (7). Markers of free radical oxidation products prove the predominance of pro-oxidant processes in schizophrenia. The most reliable proof of predominance of pro-oxidant processes is the increase in thiobarbituric acid reactive substances in drug-naive first-episode psychosis, stable medicated outpatients, and chronic inpatients, which is confirmed by the results of meta-analysis (7, 8). Prior research has also demonstrated that exposure of healthy humans to chronic intermittent hypoxia increased oxidative stress by overproduction of reactive oxygen species (ROS) (9), and intracellular ROS paradoxically increased under hypoxic conditions (10). There is an extensive literature of indirect evidence suggestive of a role for hypoxia in SCH pathophysiology. There is, for example, a strong association between fetal hypoxia and future development of SCH (11), increased expression of hypoxia-inducible factors in SCH postmortem prefrontal cortex (12), and a much higher proportion (55%) of genes associated with SCH being regulated by hypoxia and/or expressed in vasculature than would be expected by chance (13). Although there is considerable indirect evidence that hypoxia is closely related to schizophrenia, direct evidence of hypoxia in SCH has never been found. Abnormalities of oxidative stress are also known to exist in individuals with depression. In previous research, the current study's corresponding author confirmed the presence of histogenous hypoxia and an increased propensity for the accumulation of acidic metabolites in depressed patients by comparing samples of their venous blood gas (VBG) with those of healthy controls (14). The present research considers whether people with SCH also exhibit histogenous hypoxia and acid retention. The purpose of the present study is to clarify whether there are abnormal oxidative stress linked to SCH and whether histogenous hypoxia and acid retention may be present. However, the effect of pharmacotherapy on these indicators remains unclear; furthermore, the link between them and the psychiatric symptoms of SCH remain unknown. To address these unknown aspects, we designed this study to compare the SOD and VBG analysis results in SCH patients with those of a healthy control sample group, as well as a self-controlled study to compare SOD and VBG results in acute and stable SCH patients. Materials and Methods ParticipantsThe research participants were SCH patients and controls. The SCH patients were recruited from among inpatients attending the Department of Traditional Chinese Medicine in The Affiliated Brain Hospital of Guangzhou Medical University. The control group was recruited from among the staff members of the Department of Traditional Chinese Medicine and was matched approximately with the SCH patients by gender and age. The inclusion criteria for the SCH group were, first, that the prospective participant's current symptoms met the diagnosis of SCH according to the International Statistical Classification of Diseases and Related Health Problems (code F20) (15). In other words, they are in the acute phase of schizophrenia; second, that they were between 18 and 60 years old. Exclusion criteria for the SCH group were as follows: (a) presence of fatty liver disease, hypertension, gout, renal failure, diabetes, or other medical diseases; (b) abuse of alcohol and/or psychoactive substances; (c) pregnant or lactating female patients; (d) patients confined to bed or a wheelchair; and (e) infection or alcohol-intake history within the previous week. The inclusion criteria for the control group were as follows: (a) a good health record, featuring no physical illness or psychiatric disorder; (b) predominantly normal results from the most recent examination; (c) no sleeping disorders; and (d) no infection or alcohol-intake history within the previous week. Exclusion criteria for the control group were as follows: (a) presence of fatty liver disease, hypertension, gout, renal failure, diabetes, or other medical diseases; (b) abuse of alcohol and/or psychoactive substances; (c) pregnant or lactating female patients; (d) patients confined to bed or a wheelchair; and (e) infection or alcohol-intake history within the previous week. Clinical InterviewsParticipants from both groups were invited to attend a clinical interview. The SCH patients were interviewed by an experienced psychiatrist to determine whether they met the screening criteria. All participants were informed of the purpose, procedure, and possible benefits and risks of participation in this study. Participants who agreed to join the study were asked to sign informed consent forms and register their general data, encompassing the following: (a) background information, (b) demographic items, (c) previous history, and (d) medical history. Assessment of Symptom SeverityParticipants in the SCH group were assessed by an experienced psychiatrist, who used the Positive and Negative Symptom Scale (PANSS) (16) to measure the positive, negative, and general symptoms of psychopathology. This evaluation tool is widely applied in China, and its reliability and validity have been verified; the internal consistency reliability (Cronbach α) was 0.87, whereas the internal consistency reliability of the five dimensions (e.g., positive symptoms, negative symptoms, depression, mania, and cognitive impairment) ranged from 0.74 to 0.90 (17). The analysis indicators incorporated scores from the PANSS subscale pertaining to positive symptoms (PANSS-P), the subscale measuring negative symptoms (PANSS-N), and the general psychopathology subscale (PANSS-G), as well as the total PANSS score (PANSS-T). Assessment of Biochemical MeasuresElbow vein fasting blood was drawn in the morning from each participant through several tubes. Indicators included VBG analysis, SOD, and general biochemical indicators (evaluated through lipid profile and kidney function). A single-use arterial blood gas needle was used for the VBG analysis, with samples taken from the elbow vein and tested using a fully automatic blood gas analyzer within 15 min of the sample being collected. The analysis indicators included the venous pH value, the venous partial pressure of oxygen (PvO2), the venous partial pressure of carbon dioxide (PvCO2), lactic acid, potassium ions (K+), and fasting blood glucose (FBG). SOD activity was determined with method of pyrogallol autoxidation in clinical laboratory of Affiliated Brain Hospital of Guangzhou Medical University. Intervening MeasureControl group is no longer special intervening measure. The SCH group was conducted with combination treatment of atypical antipsychotics (AASs) and Chinese medicine decoction in hospital. AAS included olanzapine, clozapine, risperidone, quetiapine, and aripiprazole with routine dosage in this study. The Chinese medicine decoction was named Ditan Huayu Lishen Decoction for purging phlegm, removing blood stasis, and tranquilizing the mind, which was composed of the following ingredients: fructus aurantii immaturus, Pinellia, Poria, Curcuma longa 15 g each, and Bryozoatum 20 g, bile Arisaema 10 g, Fritillaria, Rhizoma acori graminei, peach kernel, flowers Carthami, zedoary, Hirudo, Eupolyphaga 15 g each, miltiorrhiza 20 g, licorice 10 g, Rheum officinale 15 g (decoct later), and succinum 5 g (take drenched). The recipe is suitable for various mental diseases caused by phlegm and blood stasis (18). The compositions of the recipe were soaked in 800 ml water for 15 min and then boiled with high heat and simmered for half an hour. The filtrate was taken two times every day. ReassessmentThe vein blood gas analysis, SOD, and PANSS were evaluated after 4 weeks' intervention in hospital in the SCH group. Statistical AnalysesAll statistical analyses were performed using the PASW Statistics 20.0 software package. The measured data were presented with mean ± standard deviation (SD) following the data normality test. Enumeration data were presented with the constituent rate and ratio units. The test level was set to α = 0.05. The effect size (Cohen d) was calculated with web-based effect-size calculator in https://www.campbellcollaboration.org/. Measurement DataFor normally distributed data, comparison between two groups was performed using an independent-samples t-test; comparison between before and after treatment in the SCH group was performed using paired-samples t-test. The relationship between two variables was determined through Pearson correlation analysis. The relationship between a dependent variable and multiple factors was determined using multiple linear regression analysis. Enumeration DataComparison of constituent rates and ratios between two groups was carried out with a χ2 test or Fisher exact test. Results Characteristics of the ParticipantsThere were 40 participants in the SCH group−25 males (62.5%), 15 females (37.5%)—and 40 in the control group−24 males (60.0%), 16 females (40.0%). There was no significant difference in gender balance between the two groups. In the SCH group, there were four participants (10%) who were postmenopausal; two participants (5%) held religious beliefs, but 38 cases (95%) did not; four participants (10%) reported a history of smoking; two (5%) had experienced first-episode psychosis and 38 (95%) recurrent episodes; 23 (57.5%) were receiving treatment (e.g., olanzapine, clozapine, risperidone, quetiapine, aripiprazole), whereas 17 (42.5%) were not, and two cases had never been treated. The average withdrawal period from antipsychotic treatment was 9.71 months (1, 384). The course of the disease was 102.30 ± 99.93 months. The course of Western medicine treatment was 79.61 ± 95.07 months. In the control group, three participants (7.5%) were postmenopausal; three (7.5%) held religious beliefs, and 37 (92.5%) did not; six participants (12%) reported a history of smoking. Thus, no significant differences were found between the two groups regarding the respective distribution of postmenopausal, religious beliefs, and smoking history data. The two groups featured no significant differences in terms of participants' relative ages, heights, weights, and body mass indexes (BMIs), but there were differences regarding education levels: the years of education completed by participants in the SCH group were lower than those in the control group (Table 1). TABLE 1
Table 1. Participants' ages, education levels, heights, weights, and BMIs (mean ± SD). Comparison in BaselineThe venous pH, PvO2 values, and FBG in the SCH group were higher than those in the control group, whereas PvCO2 and lactic acid levels were lower, indicating statistical significance (p < 0.001 in all). There were no significant differences in K+ levels between the two groups. The SOD levels in the SCH group were lower than those of the control group, indicating statistical significance (p < 0.001). (Table 2; Figures 1–3). TABLE 2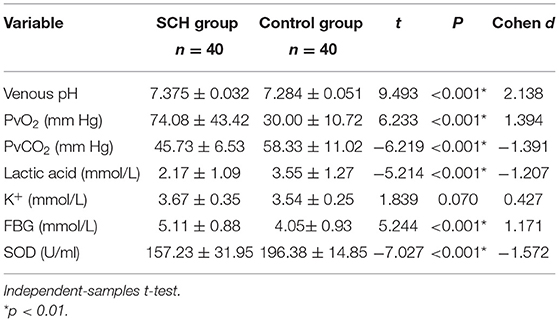
Table 2. VBG analysis and SOD (mean ± SD). FIGURE 1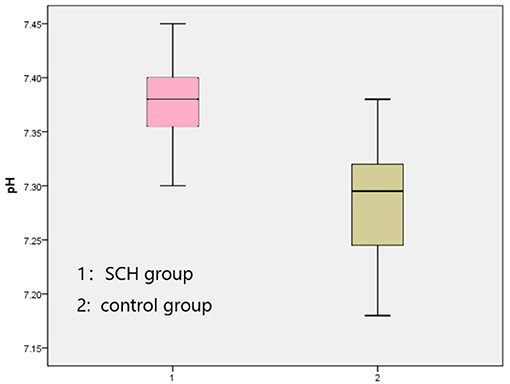
Figure 1. Comparison venous pH between two groups. FIGURE 2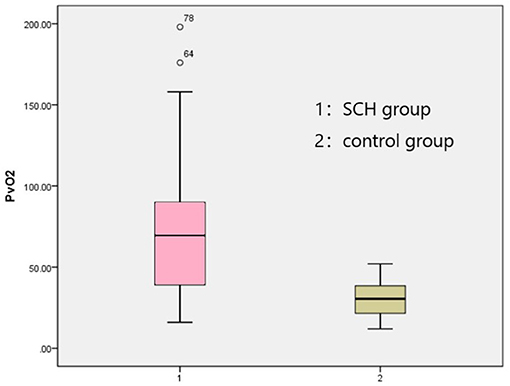
Figure 2. Comparison PvO2 between two groups. FIGURE 3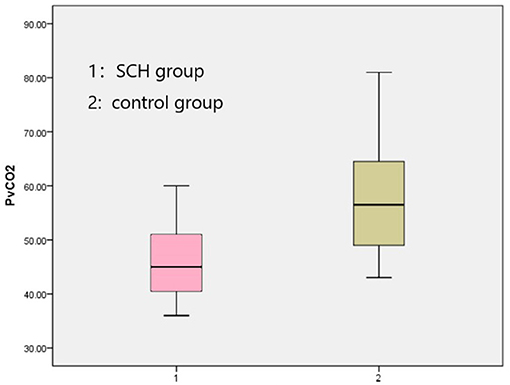
Figure 3. Comparison PvCO2 between two groups. Correlation Analyses General Condition and the Effect IndicatorsIn order to clarify whether a participant's general condition (i.e., age, education, smoking history, height, weight, BMI, first episode, course of disease, course of treatment) had an impact on any of the effect indicators, correlation analysis between them was undertaken. It was found that SOD was positively correlated with BMI, and FBG was positively correlated with course of disease and course of treatment in the SCH group (Table 3), whereas age was positively correlated with venous pH and negatively correlated with PvCO2 and SOD, education was negatively correlated with K+, and height was positively correlated with PvCO2 in the control group (Table 4). TABLE 3
Table 3. Correlation analysis between the general situation and the effect indicators in the SCH group. TABLE 4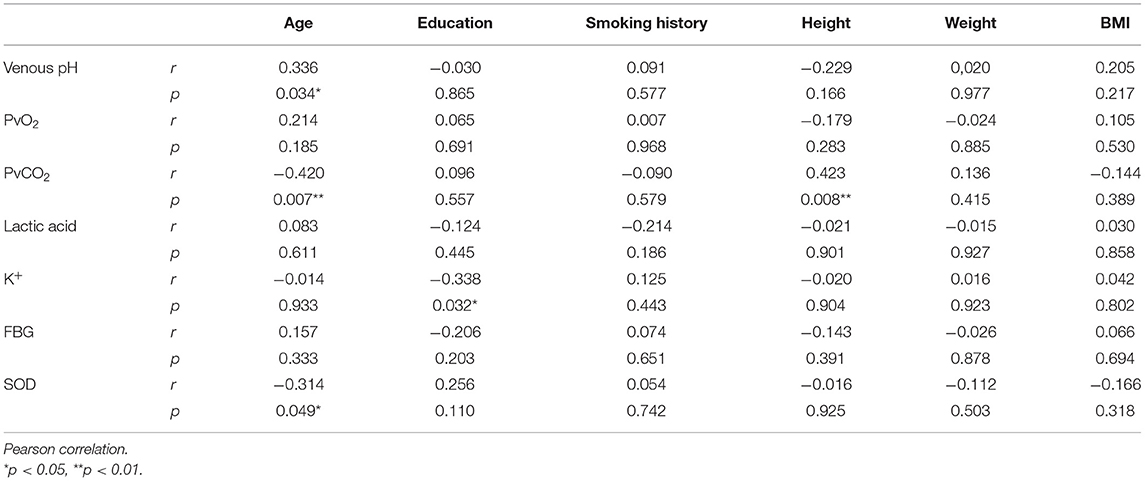
Table 4. Correlation analysis between the general situation and the effect indicators in the control group. PANSS Scores and Other Effect IndicatorsTo explore the relationship between the PANSS scores and other variables, Pearson r was measured. It was found that K+ was negatively correlated with the PANSS-G and PANSS-T score, and SOD was negatively correlated with the PANSS-G score, whereas venous pH, PvO2, PvCO2, and lactic acid were no correlated with it (Table 5). TABLE 5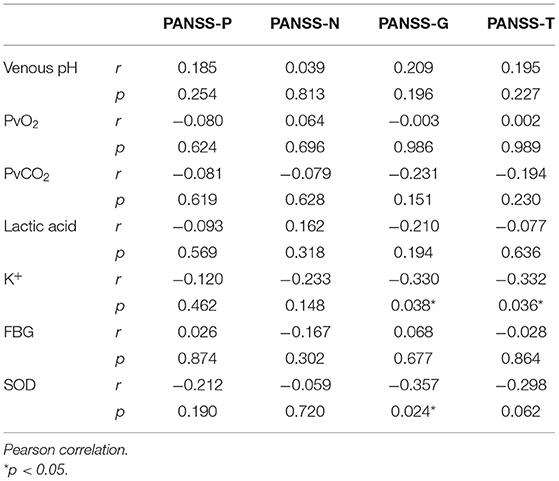
Table 5. Correlation analysis of PANSS scores and other effect indicators. Venous pH, PvO2, PvCO2, and Other Effect IndicatorsTo explore the relationship between venous pH, PvO2, PvCO2, and other variables, Pearson r was measured both in the SCH group and the control group. It was found that K+ was negatively correlated with PvO2, and lactic acid was negatively correlated with venous pH in the SCH group (Table 6), whereas FBG was positively correlated with venous pH and PvO2 negatively correlated with PvCO2, and lactic acid was positively correlated with PvCO2 and negatively correlated with venous pH in the control group (Table 7). TABLE 6
Table 6. Correlation analysis of venous pH, PvO2, PvCO2, and other effect indicators in the SCH group. TABLE 7
Table 7. Correlation analysis of venous pH, PvO2, PvCO2, and other effect indicators in the control group. Multiple Linear Regression Analysis of PvO2To explore the relationship between PvO2 and other variables, Pearson r was measured. In acute SCH patients, it was found that PvCO2 exhibited negative correlation with PvO2 (r = −0.581, p < 0.001), whereas venous pH value showed positive correlation with it (r = 0.650, p < 0.001). Based on these results, a multiple linear regression analysis was performed with venous pH and PvCO2 as the independent variables and PvO2 as the dependent variable. As a result, venous pH was the only variable remaining in the regression model (t = 5.268, p < 0.001, unstandardized coefficient = 880.699, standardized coefficient = 0.650, constant = −6,420.858). The model was tested with F(1, 38) = 27.747, p < 0.001, indicating that the venous pH was significant. Thus, the regression model could be described as follows: PvO2 = −6,420.858 + 880.699 venous pH. The absolute values of the standardized residual and the student-adjusted residual were |
【本文地址】
今日新闻 |
推荐新闻 |